Zinc Bromide And Potassium Sulfide Precipitate . See examples, net ionic equations, and tests for. calculate net ionic equation. Reaction of zinc bromide and potassium sulfide. For example, ag3po4 + hcl. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. Enter an equation of an ionic chemical equation and press the balance button. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate.
from www.numerade.com
Reaction of zinc bromide and potassium sulfide. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. For example, ag3po4 + hcl. Enter an equation of an ionic chemical equation and press the balance button. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. calculate net ionic equation. See examples, net ionic equations, and tests for. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a.
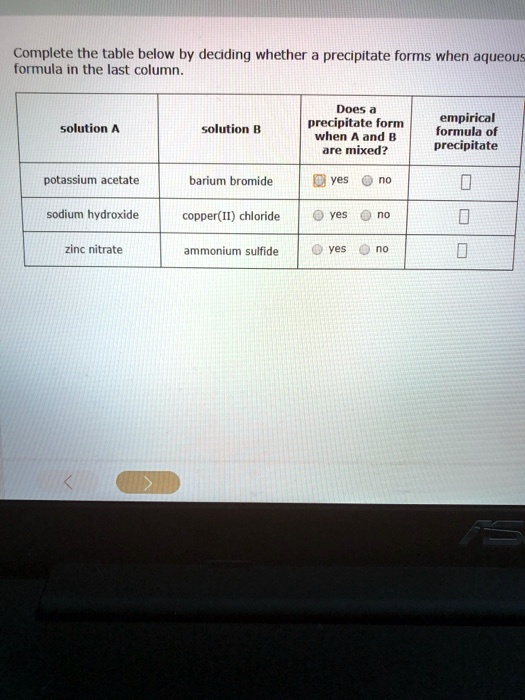
SOLVED Complete the table below by deciding whether precipitate forms
Zinc Bromide And Potassium Sulfide Precipitate calculate net ionic equation. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. calculate net ionic equation. Enter an equation of an ionic chemical equation and press the balance button. See examples, net ionic equations, and tests for. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Reaction of zinc bromide and potassium sulfide. For example, ag3po4 + hcl. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a.
From www.numerade.com
SOLVED SIMPLE REACTIONS Predicting precipitation Complete the table Zinc Bromide And Potassium Sulfide Precipitate Reaction of zinc bromide and potassium sulfide. calculate net ionic equation. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. watch this video to learn about precipitation. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Complete the table below bv deciding whether precipitate forms Zinc Bromide And Potassium Sulfide Precipitate Reaction of zinc bromide and potassium sulfide. For example, ag3po4 + hcl. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. learn. Zinc Bromide And Potassium Sulfide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Bromide And Potassium Sulfide Precipitate Enter an equation of an ionic chemical equation and press the balance button. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. See examples, net ionic equations,. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Aqueous solutions of zinc bromide and sodium phosphate combine Zinc Bromide And Potassium Sulfide Precipitate For example, ag3po4 + hcl. Reaction of zinc bromide and potassium sulfide. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. calculate net ionic equation. See examples, net ionic equations, and tests for. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Write the net ionic equation for the precipitation reaction Zinc Bromide And Potassium Sulfide Precipitate learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. For example, ag3po4 + hcl. See examples, net ionic equations, and tests for. Reaction of zinc bromide and potassium sulfide.. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Zinc Bromide And Potassium Sulfide Precipitate Enter an equation of an ionic chemical equation and press the balance button. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. See examples, net ionic equations, and tests for. calculate net ionic equation. Reaction of zinc bromide and potassium sulfide. watch this video to learn about precipitation reactions, a type of. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Texts Complete the table below by deciding whether a Zinc Bromide And Potassium Sulfide Precipitate calculate net ionic equation. For example, ag3po4 + hcl. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. Enter an equation of an ionic chemical equation and press the balance button. See examples, net ionic equations, and tests for. learn how precipitation reactions occur when insoluble salts form from aqueous. Zinc Bromide And Potassium Sulfide Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Zinc Bromide And Potassium Sulfide Precipitate See examples, net ionic equations, and tests for. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. Enter an equation of an ionic chemical equation and press the balance button. For example, ag3po4 + hcl. calculate net ionic equation. Reaction of zinc bromide and potassium sulfide. adding 10.0 ml of a dilute. Zinc Bromide And Potassium Sulfide Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Zinc Bromide And Potassium Sulfide Precipitate For example, ag3po4 + hcl. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. Reaction of. Zinc Bromide And Potassium Sulfide Precipitate.
From www.chegg.com
Solved does zinc bromide and sodium sulfide precipitate and Zinc Bromide And Potassium Sulfide Precipitate when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. For example, ag3po4 + hcl. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. calculate. Zinc Bromide And Potassium Sulfide Precipitate.
From www.slideshare.net
Precipitates Zinc Bromide And Potassium Sulfide Precipitate Reaction of zinc bromide and potassium sulfide. Enter an equation of an ionic chemical equation and press the balance button. For example, ag3po4 + hcl. See examples, net ionic equations, and tests for. calculate net ionic equation. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. watch this video to learn about. Zinc Bromide And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Bromide And Potassium Sulfide Precipitate adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED When 12.0 mL of a 6.54x10=4 M zinc bromide solution is combined Zinc Bromide And Potassium Sulfide Precipitate when two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. calculate net ionic equation. See examples, net ionic equations, and tests for. watch this video to learn about precipitation reactions, a type of chemical reaction that. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Zinc Bromide And Potassium Sulfide Precipitate See examples, net ionic equations, and tests for. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. learn how precipitation reactions occur. Zinc Bromide And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Bromide And Potassium Sulfide Precipitate calculate net ionic equation. For example, ag3po4 + hcl. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. watch this video to learn about precipitation reactions, a. Zinc Bromide And Potassium Sulfide Precipitate.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Zinc Bromide And Potassium Sulfide Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. For example, ag3po4 + hcl. adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. when two aqueous solutions of ionic compounds are. Zinc Bromide And Potassium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Bromide And Potassium Sulfide Precipitate learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. Reaction of zinc bromide and potassium sulfide. For example, ag3po4 + hcl. Enter an equation of an ionic chemical equation and press the balance button. calculate net ionic equation. See examples, net ionic equations, and tests for. watch this video to learn about. Zinc Bromide And Potassium Sulfide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Bromide And Potassium Sulfide Precipitate adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. learn how precipitation reactions occur when insoluble salts form from aqueous solutions of ions. Enter an equation of an ionic chemical equation and press the balance button. when two aqueous solutions of ionic. Zinc Bromide And Potassium Sulfide Precipitate.